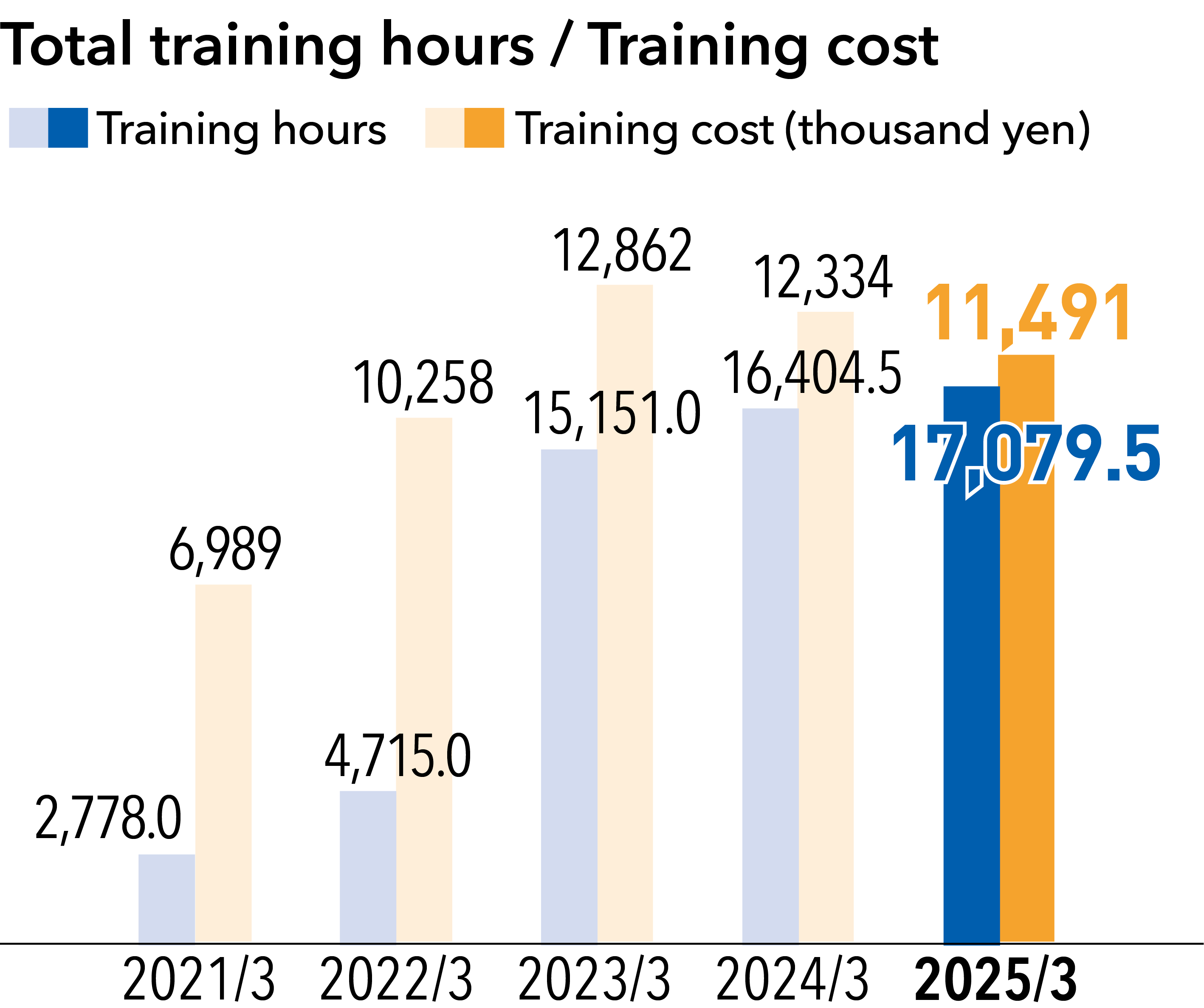The Kaga Electronics Group, guided by our Corporate Philosophy of “Everything we do is for our customers”, respects the human rights of all stakeholders impacted by our business activities, strives to realize a sustainable society through our business activities, and is working to respect human rights as part of fulfilling the social responsibility expected of us as a company, in accordance with our Basic CSR Policy and the Kaga Electronics Group Code of Conduct and Sustainability Policy.
Commitment to Respect for Human Rights
As well as prohibiting human trafficking, forced labor and child labor, the Kaga Electronics Group is promoting efforts to respect human rights in all aspects of recruitment, employment, education and training, and promotion, such as by respecting the history, culture and customs of each country and region as well as the personality and individuality of each person; prohibiting discrimination as well as physical and mental harassment on grounds such as race, creed, gender, sexual orientation, gender identity, age, religion, nationality, language, physical characteristics, disability, property and place of origin; providing a safe and comfortable working environment, protecting freedom of association and the right to collective bargaining, and ensuring fair compensation.
Support and Respect for International Norms
The Kaga Electronics Group supports international human rights standards such as the International Bill of Human Rights and the ILO Declaration on Fundamental Principles and Rights at Work. We also respect human rights in accordance with the UN Guiding Principles on Business and Human Rights.
Human Rights Due Diligence
The Kaga Electronics Group has established a human rights due diligence system to respect human rights. Through this system, we identify and address any actual or potential adverse impacts on human rights arising from our business activities, and strive to prevent and mitigate such impacts.
Remediation and Redress
In the event that our business activities have caused or contributed to adverse impacts on human rights, the Kaga Electronics Group takes appropriate measures to remedy and redress such impacts through appropriate procedures and dialogue.
Dialogue and Consultation
The Kaga Electronics Group engages in dialogue and consultation with business partners, shareholders, local communities, and other stakeholders to deepen mutual understanding of the potential impact of our Group’s business activities. Through this process, we improve our business, products, and services; strive to enhance our efforts to respect human rights; and aim to contribute to society and co-exist in harmony.
Awareness and Education
The Kaga Electronics Group conducts the necessary awareness-raising activities and education for all our executive officers and employees (including contract employees, temporary workers, and the like; the same applies hereinafter) to ensure that this Policy is appropriately incorporated and put into practice in all of our business activities.
Information Disclosure
The Kaga Electronics Group continuously monitors our efforts to respect human rights in accordance with this Policy, and we strive to ensure transparency by disclosing our progress and results on our website, etc.
Scope of Application
This Policy applies to all executive officers and employees of the Kaga Electronics Group. We also expect our business partners and other stakeholders in our business activities to comply with this Policy and to respect human rights.
SustainabilitySUSTAINABILITY
Social
Promotion Structure
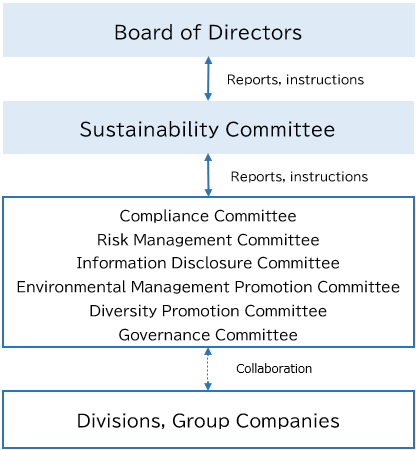
The Kaga Electronics Group recognizes the promotion of CSR and sustainability as important management issues, and have therefore established a Sustainability Committee chaired by the Representative Director, President & COO of Kaga Electronics Co., Ltd. Under the committee, we have established specialized subcommittees for Environmental Management Promotion, Diversity Promotion, Governance, Risk Management, Compliance, and Information Disclosure. This management structure promotes CSR and sustainability across the Group.
Our Group respects the human rights of all stakeholders, regardless of gender, age, nationality, social status, disability, or other personal attributes, and we are working to address human rights risks in collaboration with each specialized subcommittee. The contents of such efforts are deliberated and reported by the Sustainability Committee, and they are regularly reported to the Board of Directors.
Training and Education
The Kaga Electronics Group’s approach to human resources can be summarized in a single sentence: “People are our greatest asset.” In line with this approach, we have worked to nurture independent, autonomous, and self-motivated individuals who have a strong spirit. In the course of human resource management, we remain mindful of what can be done to maximize the value of our human resources and bring out their full potential while encouraging them to utilize their unique strengths as Kaga Electronics Group employees. Under this policy, we conduct various internal training programs to raise awareness of the relationship between corporate activities and human rights.
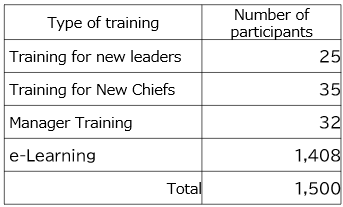
As part of our activities to enhance employee awareness of human rights, we conduct e-learning programs every year on topics such as CSR, compliance, harassment, and information security in an ongoing effort to promote a deeper understanding of these issues. We also provide human rights education as part of the training given when employees are promoted to leaders, chiefs, and managers.
In 2025, we are conducting harassment prevention training for section chiefs and higher-level managers, as well as for employees with titled positions at our overseas subsidiaries (158 participants expected in 2025). This training program aims to deepen understanding of specific examples of harassment (sexual harassment, workplace bullying, etc.) that have become a talking point in recent years, changes in laws and regulations, and practical considerations, while also raising awareness of measures to prevent harassment. In Japan, we conduct face-to-face training aimed at promoting understanding of diverse perspectives and raising awareness of human rights through peer learning.
(In addition, every December during "Human Rights Week", we post information about human rights awareness on our internal intranet to foster greater awareness among employees.)
*Peer learning is a method of learning whereby participants learn from each other through dialogue.
Occupational Safety and Health
The Kaga Electronics Group is continuously committed to managing health and ensuring workplace safety through various health checkups and consultations with industrial physicians, etc., as well as monthly meetings of the groupwide Safety and Health Committee held under the jurisdiction of the director in charge, with the aim of maintaining a workplace environment where each and every employee can work in good physical and mental health.
In addition, we conduct regular workplace inspections, employee stress checks, monitoring of long working hours, and promote efforts to identify and reduce occupational safety and health risks in our business activities in order to prevent occupational accidents and maintain an appropriate working environment.
Working Environment
Kaga Electronics conducted an engagement survey to objectively assess employee awareness, and use the results to improve understanding of the current situation and the workplace environment. Going forward, we will continue to work to create a more comfortable workplace based on the survey results.
Harassment Prevention
The Kaga Electronics Group respects the human rights of all individuals involved in our business activities, recognizes everyone as equal partners in the performance of their duties, and strives to maintain a healthy and comfortable working environment. We will not tolerate any form of discrimination on grounds such as race, creed, gender, sexual orientation, gender identity, age, religion, nationality, language, physical characteristics, disability, property or place of origin, or any form of physical or mental harassment. Our Group has established guidelines for preventing harassment, and we are continuously engaged in educational and awareness-raising activities. We are also working to establish consultation services, ensure anonymity, and develop a prompt and fair response system. We respect the human rights and privacy of those who make consultations and other related parties, and aim to create a workplace environment where everyone can work with peace of mind.
Remediation and Redress
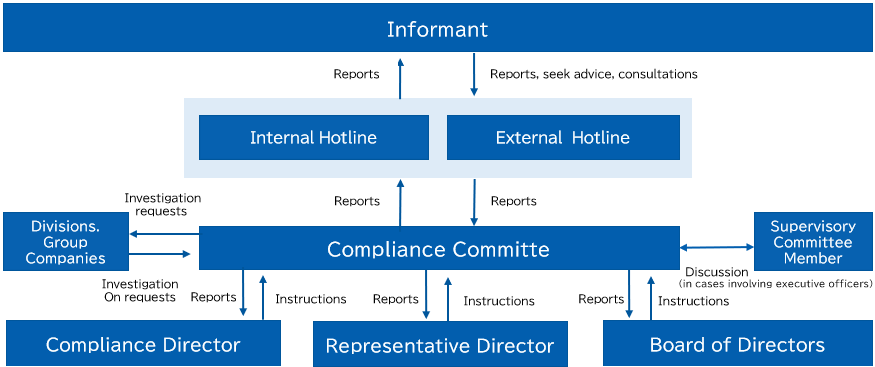
・Internal Whistleblowing System
If it becomes apparent that the business activities of Kaga Electronics or the Kaga Electronics Group have caused or contributed to adverse impacts on human rights, we will take appropriate corrective measures.
In addition, as a remedial measure, we have established an internal whistleblowing system that allows employees to anonymously report violations of laws and regulations or the Articles of Incorporation to the Representative Director, Founder & CEO, Representative Director, President & COO, Auditor, the Sustainability Committee, or the Sexual Harassment Investigation and Countermeasures Committee without the involvement of any other parties.
Reports made through the internal whistleblowing system can be submitted anonymously via the “Private Comments Box Web System”* or by telephone, email, or postal mail. Since reporting individuals cannot be identified, there is a system in place to prevent disadvantageous treatment of persons making reports.
Kaga Electronics and the Kaga Electronics Group have also established and are implementing “Internal Whistleblowing Regulations”.
*The Private Comments Box Web System is a system that allows users to anonymously submit opinions and requests to designated recipients.
This system ensures anonymity so that reporting individuals cannot be identified.
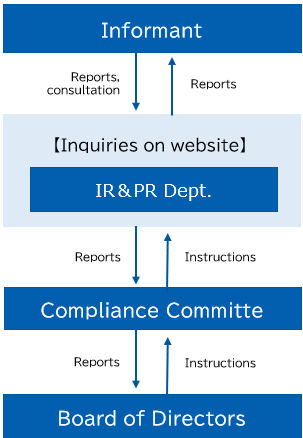
・Inquiries from the general public
We have established the following system to respond to inquiries from the general public and our stakeholders.
Implementing the idea that “everyone is a corporate manager”Human resource strategy/Diversity management
As our business activities become more global and the market environment undergoes abrupt change, diversity and taking on challenges are keys to driving sustainable growth.This is because diversity enables us to look at risk as an opportunity, and when we take on challenges we can take full advantage of these opportunities. To make this a reality, the Kaga Electronics Group promotes diversity management in terms of making effective use of diversity in human resources and in work style. At the same time, we respect our human resources who continue to take on challenges and see each person as a corporate manager. With this in mind, we will keep protecting our corporate culture of tolerating failure as a consequence of tackling challenges.
Our Basic Concept on Human Resources and Initiatives to Develop Human Resources
The Kaga Electronics Group’s approach to human resources can be summarized into a single sentence, “People are our greatest asset.” In line with this approach, we have worked to nurture independent, autonomous, and self-motivated individuals with a strong spirit. In the course of human resource management, we remain mindful of what can be done to maximize the value of our human resources and bring out their full potential while encouraging them to exercise their unique strengths as Kaga Electronics Group employees.
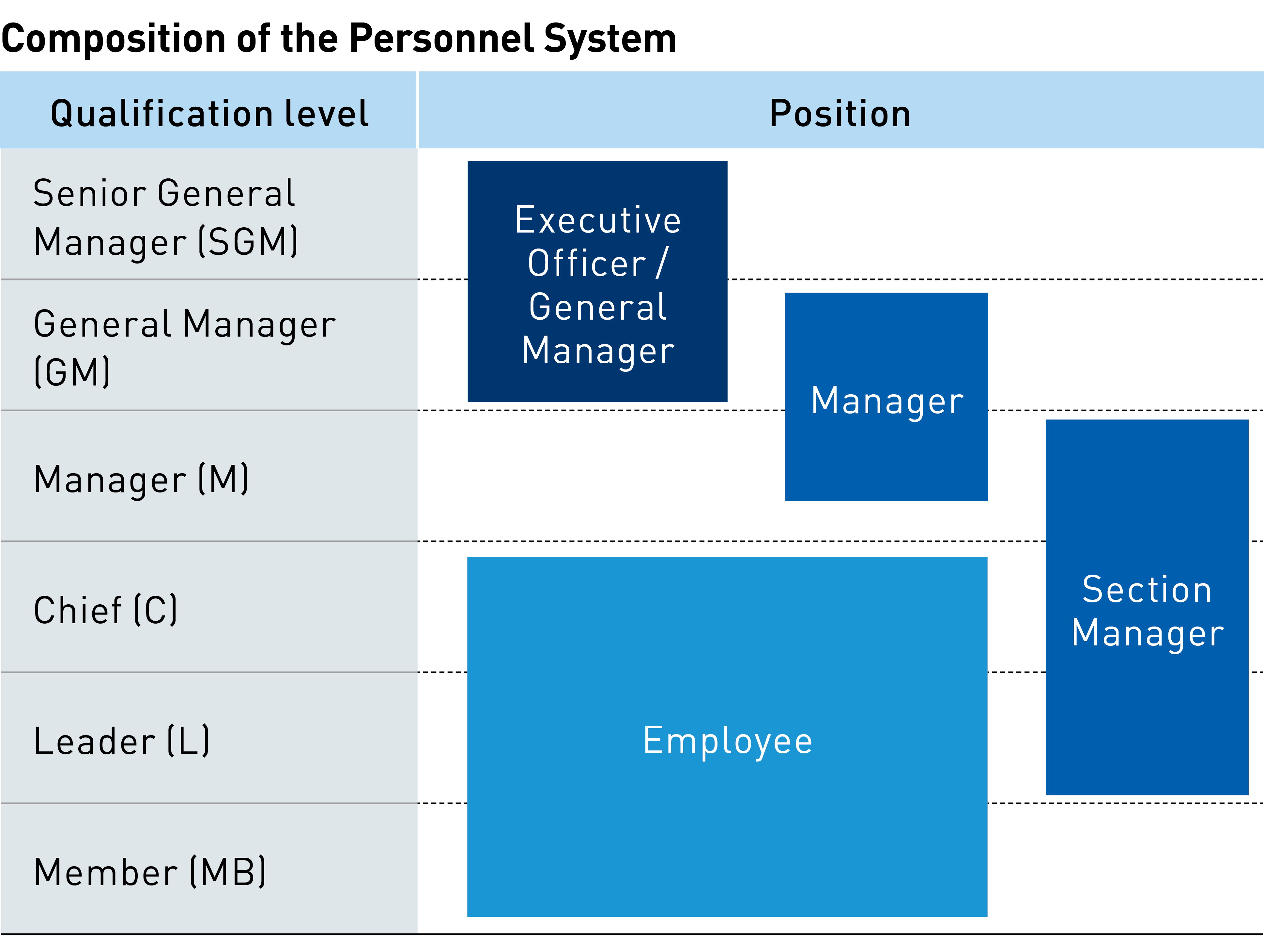
At the core of our training system are in-house position level qualification systems and training programs that differ based on employees’ years of service. The former grants membership to each employee upon joining the Group and, as they accumulate experience, promotes them to leader, chief, manager, general manager and, ultimately, senior general manager. These titles, however, do not necessarily reflect job functions.
For example, an individual at the “leader” rank could be appointed to a manager position if deemed to possess management skills. Likewise, a general manager can be appointed from among individuals who have only reached the “manager” level.
Nurturing Global Human Resources Based on the EMS Business, Which Is Expanding Overseas
With regard to the development of global human resources, the EMS Business Division serves as a hub from which employees are dispatched to overseas assignments. Newly hired graduates are assigned to the EMS Business Division, and, once they have two to three years of experience in executing the business scheme in Japan and on overseas business trips under their belts, they are dispatched. We also assign salespersons who joined the Group as mid-career hires to the EMS Business Division and then dispatch them to overseas destinations after they have amassed frontline experience for several months. Meanwhile, KAGA MICRO SOLUTION, a manufacturing subsidiary, recruits mid-career hires specialized in manufacturing, seconding them to the Production Center of the EMS Business Division once they gain proficiency. Following this secondment, they are transferred to overseas bases. Going forward, we aim to nurture human resources capable of handling overseas production management. To this end, we plan to promote similar initiatives, for example, assigning new recruits to KAGA EMS TOWADA CO., LTD. for stints at domestic factories such as Kyokuto Electric Co., Ltd. prior to being sent abroad.
To leverage the capabilities of foreign national employees, each year KAGA EMS TOWADA accepts technical trainees from Indonesia and Vietnam, taking advantage of a government-sponsored foreign technical intern training program. These trainees are given the option of either returning to their home countries after a three-year training period or obtaining a “specific skill” certification and continuing their career at KAGA EMS TOWADA. Meanwhile, Kyokuto Electric has several foreign engineers on its rolls, and in addition to quality-control work and interpretation, is actively involved in the training of its technical interns.
In terms of the promotion of foreign employees, in April 2023, Zhen Zhou Zhu, China Supervisor, was appointed as the Company’s first foreign executive officer.
Initiatives to Promote Diversity
In terms of women’s empowerment, we are making steady progress toward achieving the goals set forth in our Medium- to Long-Term Sustainability Management Plan to increase the consolidated ratio of women in managerial positions to 15% by 2024 and 17% by 2029. However, when looking at KAGA ELECTRONICS on a non-consolidated basis, along with its domestic Group companies, this ratio is not steadily growing. With this in mind, we have formulated targets aligned with the situation of each Group company. Furthermore, we are assiduously promoting initiatives to raise the ratio of women newly hired as career track employees to 30%. We recognize a need to focus on the nurturing of our female employees at a robust pace at KAGA ELECTRONICS and other domestic companies.
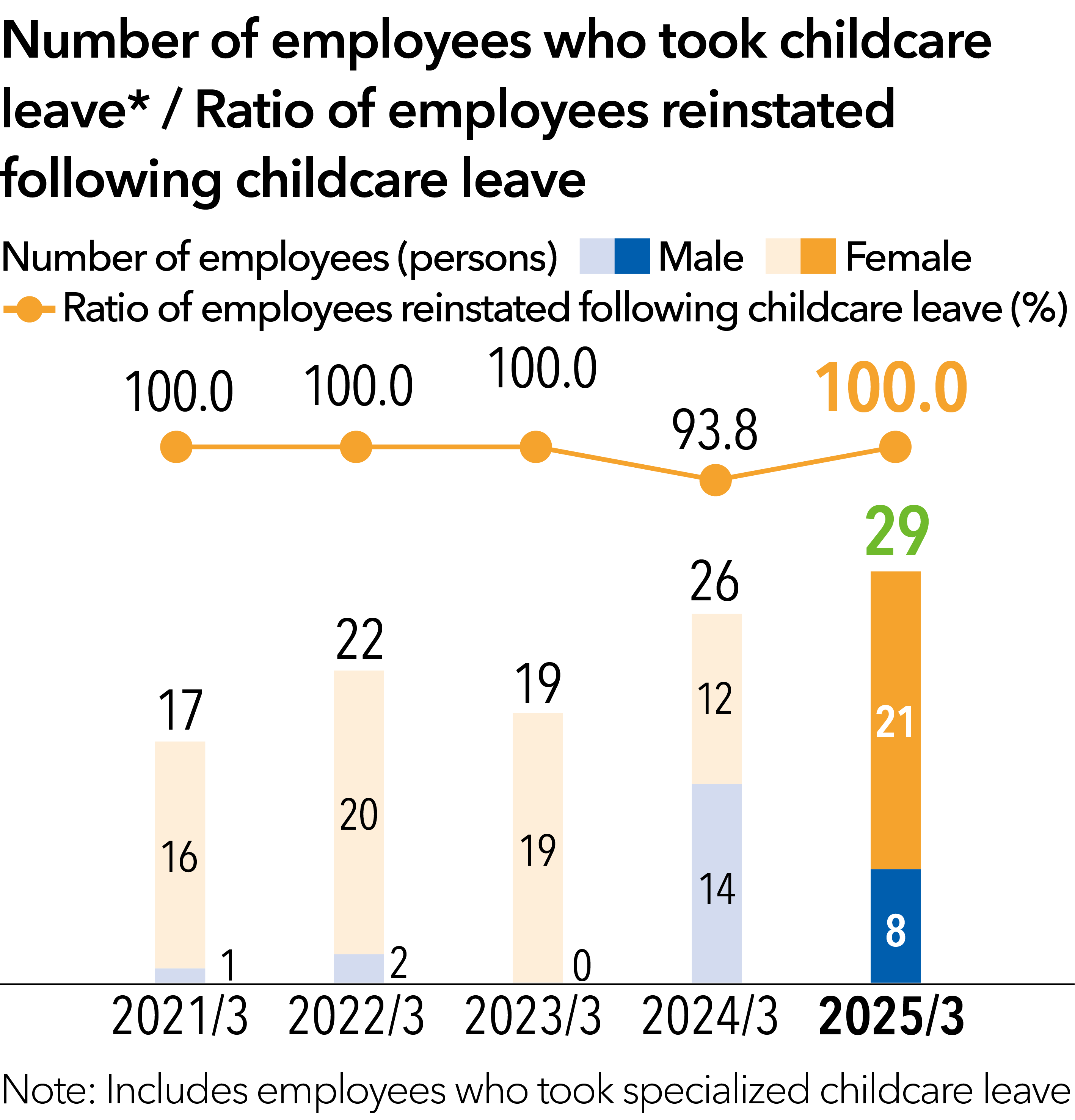
As an investment in human capital, in addition to a sharp increase in the number of male employees utilizing the new childcare leave system, the Company also implemented a Groupwide wage increase in March 2024.
Pursuing a comfortable working environmentEmployee education, training and income compensation programs
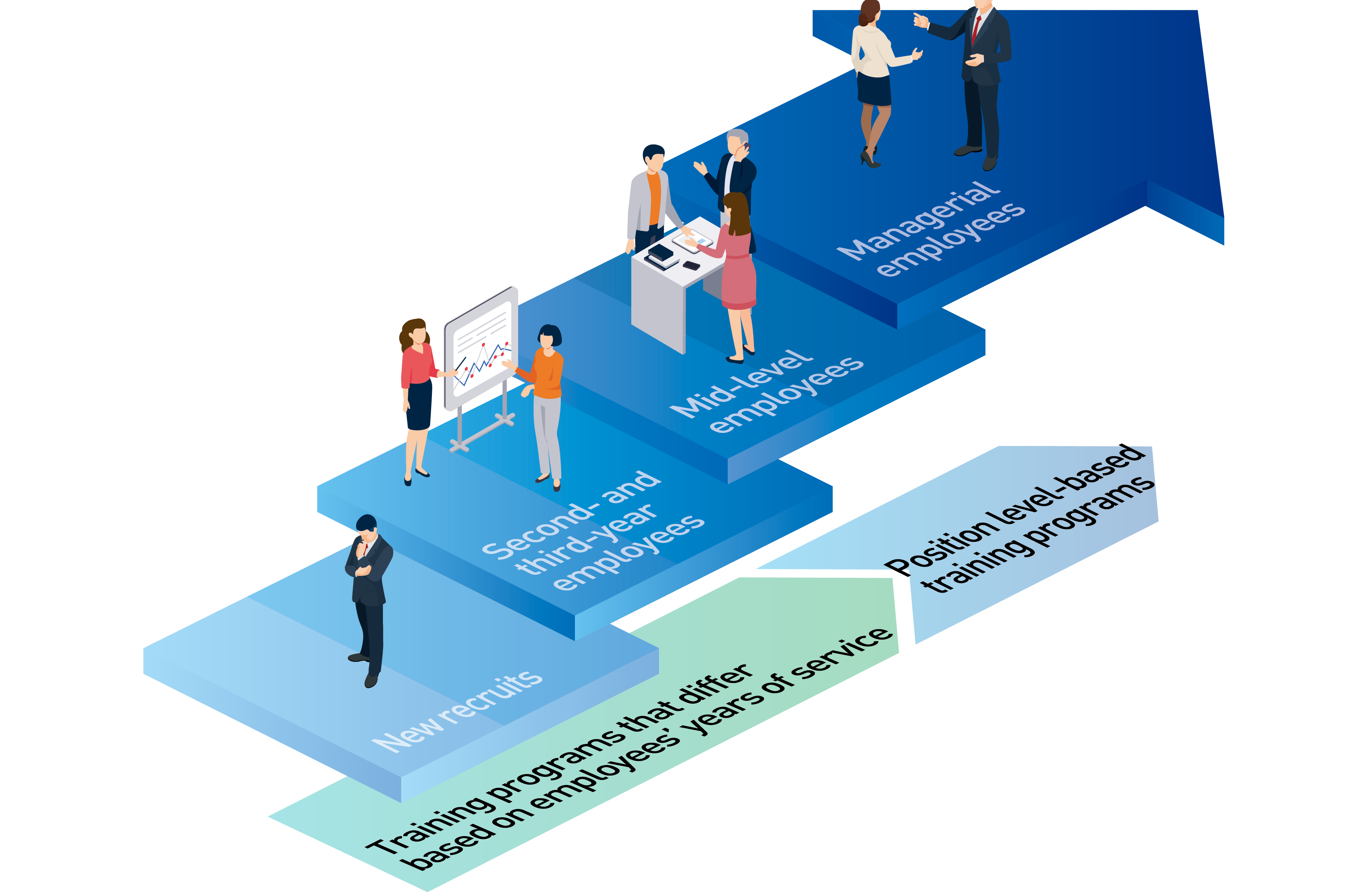
At the Kaga Electronics Group, we support the career development of employees through training for new recruits, annual training, rank-based training, e-learning, and other forms of education, as well as by providing assistance through correspondence courses.
At the core of our training system are in-house position level qualification systems and annual training programs. Under this system, every new employee receives training to qualify as a Group member before advancing to leader, chief, manager, general manager and, ultimately, senior general manager. Our training programs, which differ based on employees’ years of service, provide comprehensive support, including post-employment training for new hires, sales mindset training, factory tours (manufacturing training), six-month follow-up training, and basic technical study sessions. Over their second to fifth year, employees undergo training on a yearly basis. Around their sixth year, employees typically reach the “leader” level of positions, making them eligible to participate in such position level-based training programs as leadership training.
In addition, every year, we grant a total of six days of paid “refresh leave,” which is separate from annual paid leave, to all employees. We also instituted a sick leave program for those who develop one of three specified types of serious disease (cancer, cerebral stroke, or myocardial infarction) to help them secure their income during their hospitalization and absence from work as well as hourly paid leave. Furthermore, to encourage male employees to utilize childcare leave, we implement various initiatives—including a specialized childcare leave system that was established on April 1, 2023. Through such initiatives, we aim to strike a good work-life balance and thus realize a safer work environment for employees.
Health Management Policy
Based on our corporate philosophy, “Everything we do is for our customers,” we aim to be the No. 1 company in the industry in Japan and to become a competitive world-class company.
To this end, it is essential that each and every one of our employees is healthy in mind and body and that we are a team of professionals with integrity and high professional ethics.
We also believe that an energetic corporate culture based on a work environment that allows for smooth communication, job satisfaction, and the health of employees and their families is extremely important for the sustainable growth of a company and that the value of our existence can be enhanced by continuing to maintain and improve the health of our employees.
We will continue to implement various health promotion initiatives to encourage all employees to pursue health maintenance and improvement of their own volition.
Promotion System
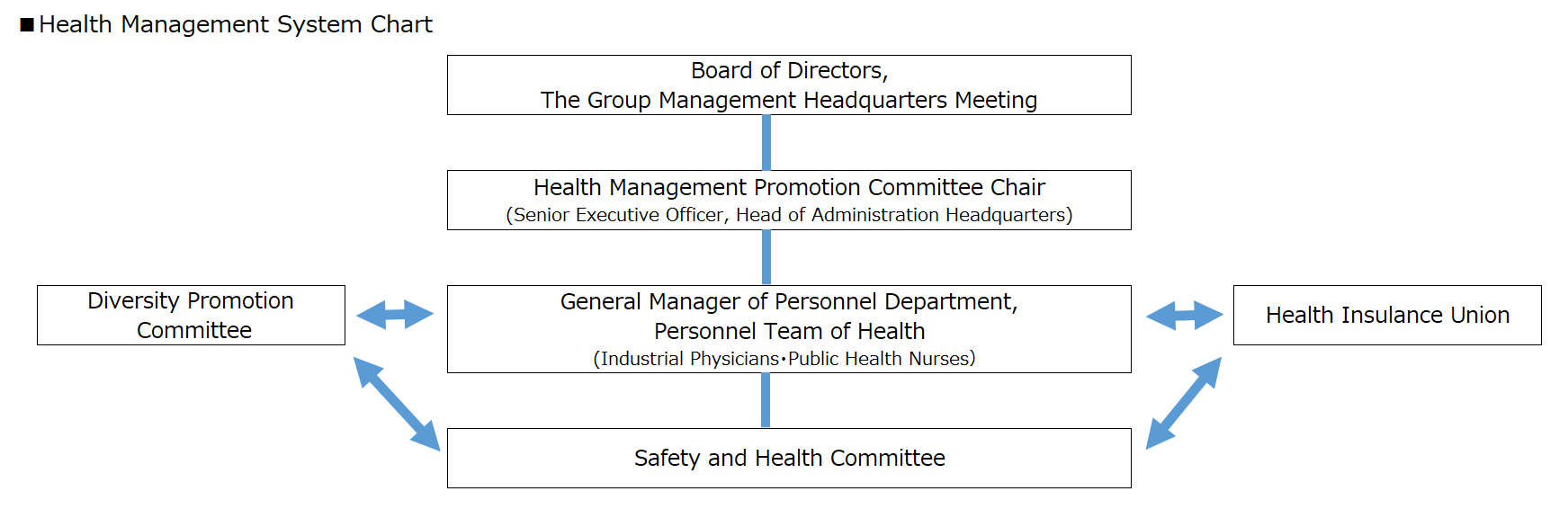
The Company has organized the "Health Management Promotion Committee" and is implementing health management initiatives. The committee is chaired by the Senior Executive Officer Head of the Administration Headquarters. It is supervised by the Board of Directors under the Group Management Headquarters Meeting, and a system is in place for reporting the content of each meeting. Under the chairperson, the General Manager of the Personnel Department and the Personnel Team, industrial physicians, public health nurses, the Diversity Promotion Committee, and the Safety and Health Committee work together to set targets in consideration of annual health issues and implement each measure.
- The Diversity Promotion Committee is a Committee that discusses and develops plans for promoting diversity, reforming working style, and health management.
Health Management Initiatives
- Prevention of mental health disorders
- We will implement measures for the mental health of employees so that they can maintain their mental health and continue to work with job satisfaction and vigor.
- <Aiming to Reduce Absenteeism>
- Absenteeism 2024/3 0.72%
- Absenteeism refers to time off work due to health problems.During the year ended March 31, 2024, 4 out of a total of 549 eligible employees took one month or more off work due to illness, representing a proportion of 4÷549=0.72%.
- Promotion of work–life balance
- We will strive to promote a work–life balance so that employees can maintain their mental and physical health and can spend quality time with their families and friends.
- <Main Initiatives>
- Promoting the use of paid leave and enhancing the hourly paid leave system
- Establishing an in-house fitness gym
- Inviting expert training coaches to implement internal consultation and training support
- <Aiming to reduce of losses due to presenteeism>
- Presenteeism 2024/3 94.5%
- Presenteeism refers to continuing to undertake work (duties) despite health problems.
- During the year ended March 31, 2024, we conducted the presenteeism survey WLQ-J (a Japanese language version of the Work Limitations Questionnaire developed by Tufts University) on 576 employees eligible for stress checks (including contract employees and others). As a result, we found an average level of presenteeism among employees equal to 94.5%.
- The benchmark level is 94.0%, with levels lower than this considered to indicate reduced employee performance and productivity.
- Presenteeism 2024/3 94.5%
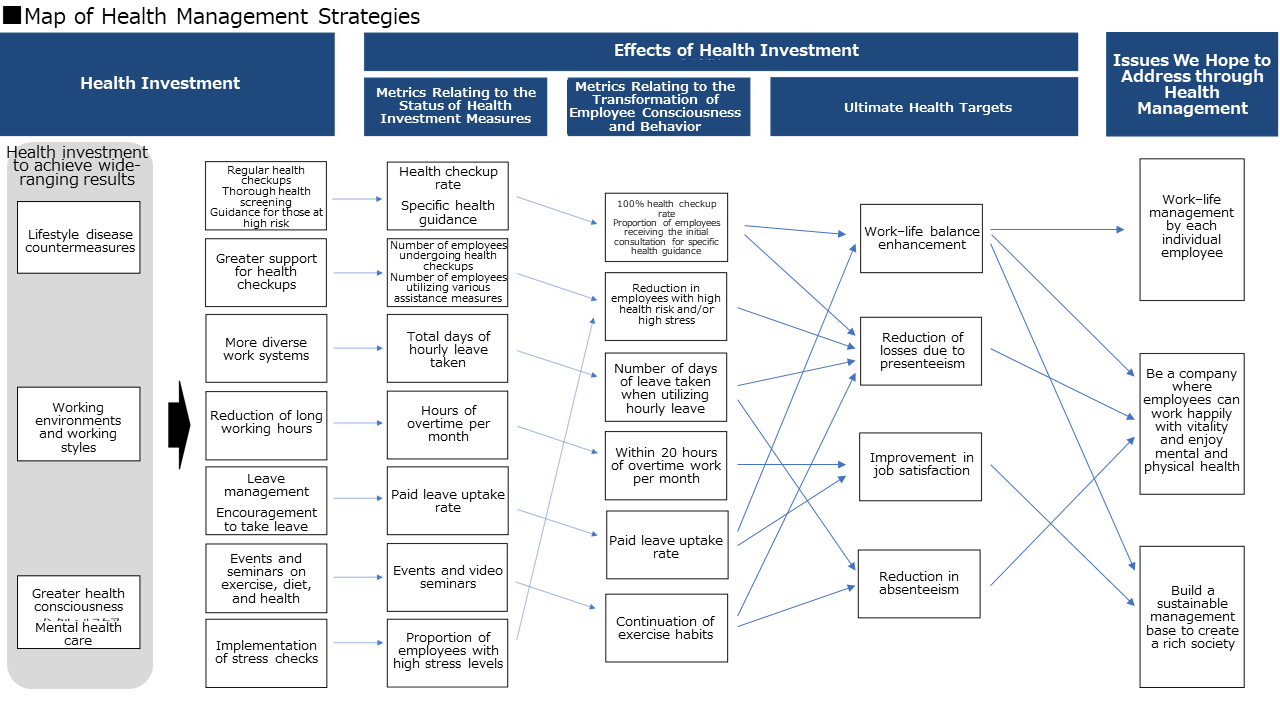
Issues We Hope to Address through Health Management
- Initiatives for the prevention of lifestyle-related diseases and for the early detection of diseases
- To understand the prevalence of various risks related to obesity, high blood pressure, and hyperglycemia, which are lifestyle-related diseases, we will work to further improve the company’s welfare system for the prevention of lifestyle-related diseases and for the early detection of cancer and other diseases.
- <Quantitative Targets>
- [Glucose metabolism] HbA1c NGSP
- Employees with a result of 10.0 or above
- ⇒100% attendance at hospital
- *HbA1c 10.0% or above (NGSP) represents the level at which restrictions become necessary to ensure stable employment due to the high risk of brain and heart disease and sudden death.
- [Blood pressure]
- Employees with a systolic blood pressure of 180 or above and/or diastolic blood pressure of 110 or above
- ⇒100% attendance at hospital
- *Systolic blood pressure of 180 or above and/or diastolic blood pressure of 110 or above (Grade 3 hypertension) represents the level at which restrictions become necessary to ensure stable employment due to the high risk of brain and heart disease and sudden death.
- Increase the proportion of employees receiving specific health guidance for the first time
- 10.5% ⇒ 18% or above
- *Specific health guidance is a form of health support provided to persons considered at high risk of lifestyle diseases based on the results of specific diagnosis and who are expected to benefit significantly from lifestyle improvements. At the initial consultation, a doctor, public health nurse, dietitian, or other health professional provides patients with support in lifestyle improvement based on factors such as each individual’s state of health and living environment.
- To address our targets for health management, we will work with industrial physicians and public health nurses to enhance our systems to promote occupational health while also collaborating with health insurance organizations to encourage employees to improve their own health.
Recognized as a 2025 Certified Health & Productivity Management Outstanding Organization
We believe that it is essential for each and every employee to be healthy both mentally and physically, and to operate as a group of professionals with integrity and high corporate ethics. We also believe that an energetic corporate culture centered on a work environment that allows for smooth communication, job satisfaction, and the health of our employees and their families is extremely important for the Company’s sustainable growth and that the value of our existence can be enhanced by continuing to maintain and improve employee health.
Accordingly, we have implemented various initiatives, including organizing a Health Management Promotion Committee and analyzing employee health checkup results to identify high-risk individuals and develop targeted approaches. For these efforts, we were recognized as a 2025 Outstanding Organization of KENKO Investment for Health (Large Enterprise Category), the third consecutive year of our receiving such recognition.

*Please see here for specific data on health management.
Basic Approach
The Kaga Electronics Group considers it our vital responsibility to provide an environment where every employee can work safely, securely, and in good physical and mental health. We have established a system for occupational safety and health management based on strict compliance with laws and regulations, and are committed to creating a healthy and comfortable workplace while striving for continuous improvement.
Structure and Initiatives
Our Group’s Safety and Health Committee meets once a month under the oversight of the Director in charge of the Health Management Promotion System to discuss issues and improvement measures related to occupational safety and health across our entire organization. We also strive to maintain employee health and improve the workplace environment through measures such as regular health checkups, various special health examinations, consultations with occupational physicians, and stress checks.
● Prevention of long working hours
To prevent excessive overtime, we monitor the overtime work status of all employees each month and share this data with the head of each department. This enables us to establish mechanisms that lead to early corrective action and strengthen working hour management for each organization. We also established recommended paid leave days starting in FY2019 to encourage planned vacation time as an initiative to promote work-life balance. In addition, by introducing an hourly paid leave system and a remote working system to support diverse work styles, we are advancing the creation of an environment where each employee can work comfortably.
● Maintaining and improving the work environment
To prevent workplace accidents and create a safe workplace, we continue to conduct regular workplace inspections, implement stress checks, and identify and reduce occupational safety and health risks.
Additionally, we conduct engagement surveys to gauge employee perceptions of the workplace environment. Our FY2025 workplace environment Diffusion Index (DI) score was 65.3, indicating a favorable overall survey trend.
● Education and awareness activities
We continuously provide education and training to enhance safety awareness and prevent accidents.
・Safe driving training
・Activities to eradicate drunk driving (utilizing breathalyzers)
・Evacuation drills and first aid training
・Installation of AEDs and training on their use
Based on the results of these initiatives, the Kaga Electronics Group will continue to strive toward creating a more comfortable workplace environment where employees can work in good physical and mental health.
Utilization of welfare facilities
With the aim of maintaining and improving the health of employees as well as providing them with a better work-life balance, in addition to resort facilities in Japan and overseas and contract golf courses, the Kaga Electronics Group also owns company retreat centers in Hakone, Karuizawa, Atami, and Kyoto (Miyazu City) as well as two cabin cruiser yachts (Yokohama and Kyoto) as company-owned welfare facilities. These facilities are widely used, with an average utilization rate of 66.5%*. We are making efforts to create a comfortable working environment to help enrich the recreational environment for employees.
Moreover, in August 2023, we opened a training room for employees in the Kaga Electronics Head Office building that boasts the latest in AI-equipped machines that help ensure optimal training tailored to each employee’s physical characteristics through the use of electronic tags, and, in June 2024, we opened a similar training room in the Head Office Building Annex. We will continue to implement various health promotion initiatives so that all of our employees can voluntarily maintain and improve their health.
* Utilization rate reflecting frequency of facility occupancy on days preceding Sundays or national holidays from April 2023 to March 2024.

A Company-owned cruiser yacht 
Training room(Kaga Electronics Head Office) 
Training room(Annex)
Purpose of these Green Procurement Guidelines
Kaga Electronics (the “Company”) is committed to management that benefits the environment considering global environmental and biodiversity conservation. As part of this commitment, we are promoting "green procurement" to contribute to the realization of a sustainable society through the reduction of environmental impact.
The Green Procurement Guidelines (the “Guidelines”) outline the green procurement standards, which represent our basic stance on green procurement. By presenting these standards, the Guidelines aim to clearly define the specific details of our requests for supplier cooperation regarding components, materials, units, products, secondary materials, and other items (“supplies”). They also aim to ensure compliance with environmental laws and regulations and mitigate the environmental impact across the entire supply chain, including reducing CO2 emissions (greenhouse gases) and promoting the use of renewable energy.
The Company’s Stance on Green Procurement
The Company considers our basic principle of green procurement to be the procurement of supplies with a small environmental impact from suppliers that actively promote environmental conservation.
[1 Priority Procurement]
As part of the initiatives we are promoting under management that benefits the environment, the Company has obtained ISO 14001 certification and puts it into practice. When procuring supplies, we prioritize suppliers who actively implement initiatives for environmental conservation.
The Company’s Management Standards for Chemical Substances in Products
The Company’s Management Standards for Chemical Substances in Products
[1 Scope of Application]
The Guidelines apply to all components and materials (components, materials, units, products (including OEM/ODM products), and secondary materials) procured by the Company for use in electric and electronic products. Additionally, in the case of supplies outside of electric and electronic products (toys, medical devices, automotive products, etc.), cases when separate standards are presented by clients of the Company, etc., the Company may request measures that correspond to management standards that differ from the content of the Guidelines.
[2 Substances Prohibited from Use in Supplies (Prohibited Substances)]
"Managed substances" (Refer to Appendix 1 of the Standards) are those whose use in supplies must be managed. These substances comply with the IEC 62474 international standard (*1) and are categorized into "Prohibited substances" and "Declarable substances."
Prohibited Substances are those listed as “prohibited” in the List of Managed Substance Groups (refer to Appendix 1 of the Standards), and their use above the threshold value is prohibited.
The Company’s prohibited substances are equivalent to the standards of International Standard IEC 62474 (*1), and consist of chemical substances whose use is prohibited or restricted under laws and regulations in Japan and overseas.
However, that the use of substances stipulated in the “RoHS Directive of the EU (2011/65/EU)” is possible for applications where it is stipulated that the directive does not apply. (See Appendix 5 of the Standards.)
Declarable Substances are those listed as "mandatory for reporting" in the List of Managed Substance Groups. This reporting requirement does not restrict the intentional use of these substances. However if they are contained in concentrations that exceed standard values, the parts that use these substances and the amount contained should be ascertained. Additionally, they should also be appropriately managed from the perspective of considerations when products are recycled and the environmental impact at the time of disposal.
If substances subject to reporting are contained in supplies, please report content information, such as the amount contained, parts that use substances, etc., in a “chemSHERPA – AI File” (*2), etc.
Requests to Suppliers
In order to promote green procurement, the Company requests the following from suppliers, who are our business partners.
(1) Submission of receipts, etc., and conclusion of agreements to the effect that suppliers will comply with the Guidelines
(2) Creation and assessment of systems for managing chemical substances in products by suppliers
(3) Conclusion of agreements to ensure the environmental quality of supplies
(4) Submission of documents related to information on chemical substances in supplies
We request that our suppliers understand and cooperate with the intent of these requests.
In cases where there are separate requirements from clients of the Company, the Company may request measures that correspond to requirements from clients.
[1 Submission of Receipts, etc., and Conclusion of Agreements to the Effect that Suppliers will Comply with the Guidelines]
The Company requests the submission of receipts, etc., after receiving the Guidelines of the Company, to confirm the agreement to comply with the content of the Guidelines. In order to ensure the environmental quality of items procured, the Company may also request the conclusion of agreements, memoranda, statements of agreement, etc., concerning chemical substances in products. (This content may also be included in basic agreements, business agreements, etc.)
When there are separate clauses related to chemical substances in products, the separate specifications shall be prioritized. The Company shall take due care with regard to the confidentiality of information submitted.
[2 Creation and Assessment of Systems for Managing Chemical Substances in Products]
The Company requests that our suppliers establish systems for managing chemical substances in their products. Additionally, to verify the establishment of these systems, we conduct assessments of suppliers' chemical substance management practices.
In principle, the Company requests that the management of chemical substances in products supplied conforms with the “Guidelines for the Management of Chemicals in Products” issued by JAMP (*3). The Company assesses management systems based on the results of self-audits conducted by suppliers or audits conducted by the Company, using materials such as the “Guidelines for the Management of Chemicals in Products (Edition 4.0) Annex Check Sheet (Version 4.01)” issued by JAMP.
[3 Submission of Documents Related to Information on Chemical Substances in Supplies]
The Company requests that suppliers survey the chemical substances in supplies and submit the following documents:
○Documents that must be submitted
・Statement proving the non-use of prohibited substances
・Information on the content of substances from the managed substance groups in supplies
(submitted via chemSHERPA – AI File, etc.)
○Documents whose submission may be required depending on the type of supplies and necessity:
・ ICP analysis data (substances restricted under the RoHS Directive)
・ MSDS, MSDS plus, component tables, mill sheets
・ Other, documents required by the Company’s clients, etc
[4 Acceptance Management]
When accepting supplies, please confirm and record that the supplies satisfy the management standards of the Company. It is also important to clarify the method of confirmation at the time of acceptance. (Evaluation method, recording method for evaluation results, identification management method, etc.)
[5 Shipping Management]
Before shipping supplies, please check that the standards for managing chemical substances in the products to be shipped have been satisfied, and record the results of this check.
As part of the acceptance and manufacturing process, please reconfirm that all predetermined checks have been performed, and manage product warehouses to ensure there are no mistaken shipments or contamination.
[6 Change Management]
If there are any changes to the following information on chemical substances in supplies, please immediately notify the Company, and make the changes after first acquiring information concerning chemical substances in products and checking compliance with management standards, submitting this information to the Company, and obtaining permission from the Company in advance.
(1) Changes to the raw materials or components used
(2) Changes to the suppliers of raw materials or components used
(3) Changes to manufacturing locations or contracted manufacturers
(4) Changes to manufacturing methods
(5) Other changes that may impact the information on chemical substances content.
[7 Management of Non-conformance]
In the event of any non-conformance in the information on chemical substances in supplies, the Company requests that suppliers promptly notify the Company and related parties, clarify any non-conforming lots, suspend shipments and prevent distribution of these lots, and perform the following management of non-conforming items.
(1) Immediately notify the Company of any non-conformities
(2) Identify, separate, suspend shipments, and prevent distribution of non-conforming items (including lot tracing)
(3) Assess non-conforming items and formulate measures for rectification
(4) Formulate measures to prevent similar incidents in advance
(5) Submit and retain related records (reports)
[8 Traceability]
In order to perform management in a way that ensures the following items, etc., can be traced from products shipped, please create records, such as lot management record slips and production record slips, and manage the history of products.
(1) Constituent components and information on chemical substances in those products
(2) Manufacturing dates and manufacturing lot numbers
(3) Manufacturing plants and contracted external parties
[9 Document and Record Management]
Please systematically manage requirements from the Company related to the management of chemical substances content, as well as information on chemical substances content and management records obtained from suppliers, by organizing related files or electronic media and creating lists of documents.
In principle, please retain the latest version of requirements from the Company, and ensure that they are available for viewing by related departments, through their distribution, the internal intranet, etc.
The retention period for documents is 10 years.
* Under the revised RoHS Directive (Directive 2011/65/EU), documents shall be retained for 10 years.
[10 Environmentally friendly initiatives]
(1) Our initiatives for global environmental conservation
The Company formulated its "Medium- to Long-Term Sustainability Management Plan" in 2021, establishing policies, measures, and targets for ESG issues that the group should address. We work as a united group to promote sustainability management to achieve these goals. In particular, we are working to provide environmentally friendly products and services. We are also striving to understand our energy consumption through business activities, reduce CO2 emissions, and increase our use of renewable energy. Additionally, we give due consideration to the importance of biodiversity. Through these initiatives, we are committed to playing an active role in realizing a society that values the global environment.
Therefore, we request that our suppliers also actively engage in environmental and biodiversity conservation through their business activities.
a) Understanding energy consumption
b) Promoting renewable energy use
c) Reducing CO2 emissions
d) Conserving biodiversity
(2) Encouragement of upstream suppliers in the supply chain
To promote efforts to reduce our environmental impact across the entire supply chain, we request that our suppliers encourage their upstream suppliers to comply with the Guidelines.
(3) Item regarding supplies - Sustainable raw material procurement
The Company is actively promoting sustainability-focused initiatives when procuring raw materials. In the procurement of natural resources, we not only comply with all relevant laws and regulations, but also take into consideration the impact on the local environment and community, and promote the use of recycled materials. In this way, we are committed to striking a balance between sustainable use of resources and their stable procurement.
We request that our suppliers take the following into consideration when supplying raw materials.
a) Raw materials produced in compliance with the laws and regulations of the production region.
b) Raw materials produced in a way that ensures safety and hygiene in the working environment.
c) Consideration is given to the impact on the local environment and ecosystem arising from raw material extraction and other processes.
d) Consideration is given to the human rights of workers and the impact on local communities, arising from raw material extraction and other processes.
e) Consideration is given to ensuring that raw materials and products do not contain conflict minerals (3TG: tin, tungsten, tantalum, gold) that are illegally mined or extracted.
Quality control system
Within its EMS Business Division, the Group established the Production Center as its headquarters. Centered around three core factory facilities—Japan (KAGA EMS TOWADA CO., LTD.), China (KAGA TAXAN (SUZHOU) ELECTRONICS CO., LTD.), and Thailand (KAGA ELECTRONICS (THAILAND) CO., LTD.)—the Group works to strengthen production technology and quality control across the entire organization. This is achieved through the coordinated efforts of its 21 EMS in-house factory locations across various regions, including the Americas, Europe, Asia/ASEAN, China, and Japan.
In addition to actualizing our commitment to “putting quality first and manufacturing products that satisfy our customers,” we have established working groups to handle quality assurance, production control, production technology, IT-based production innovation, global procurement, SDGs, etc., at every site with the overarching purpose of sharing and standardizing various indicators and standards across the Group. Furthermore, not only do we pursue such lateral development as the supplementation of know-how and resources, we also conduct regular inter-site business trip exchange meetings in order to improve quality and customer service.

A Production Center-led exchange of overseas engineers
Efforts to enhance quality
The Company’s Engineering Department controls the technological resources of the entire Group, works to ensure the effective use and optimization of these resources as well as to strengthen technological capabilities and expand business across the organization. The Systems Engineering Department collaborates with the Company and its Group companies to handle everything from planning and development to design, manufacturing, maintenance, and operation of customer products. The Quality Assurance Department seeks to enhance development quality and manages safety standards throughout the Group. This department works with other departments responsible for quality within the Group, depending on the circumstances, to resolve issues.
Prototype evaluation using a measurement device
Status of ISO9001 certification
KAGA ELECTRONICS and the following Group companies have attained ISO9000 series certification, an international standard for qualitymanagement.
The Company is striving to further enhance customer satisfaction through the continuous improvement of its quality management system.
Japan:
KAGA ELECTRONICS CO., LTD., KAGA TECH CO., LTD., AD DEVICE CO., LTD., KAGA MICRO SOLUTION CO., LTD., KAGA FEI Co., Ltd., NV DEVICES CO., LTD., KAGA EMS TOWADA CO., LTD., Kyokuto Electric Co., Ltd., KAGA TECHNO SERVICE CO., LTD.
Overseas:
<China>
KAGA (H.K.) ELECTRONICS LIMITED/ KAGA (SHENZHEN) ELECTRONICS LTD./SUZHOU TAXAN KAGA TRADING CO., LTD./ KAGA TECHNOLOGY (SUZHOU) ELECTRONICS CO., LTD./ HUBEI KAGA ELECTRONICS LIMITED
<Asia/ASEAN>
KAGA COMPONENTS (MALAYSIA) SDN. BHD./ KAGA ELECTRONICS (THAILAND) CO., LTD./PT. KAGA ELECTRONICS INDONESIA/ KAGA ELECTRONICS (VIETNAM) CO., LTD./ KAGA ELECTRONICS INDIA PRIVATE LIMITED
<The Americas>
TAXAN MEXICO S.A. DE C.V./ KAGA FEI AMERICA, Inc.
<Europe>
KD TEC s.r.o./ KD TEC TURKEY EL
The Kaga Electronics Group is promoting the creation of new businesses based on a desire to resolve social issues in a range of fields, including daycare, welfare and nursing care, to drive sustainable growth. Through our businesses, we also aim to contribute to the achievement of the Sustainable Development Goals (SDGs).

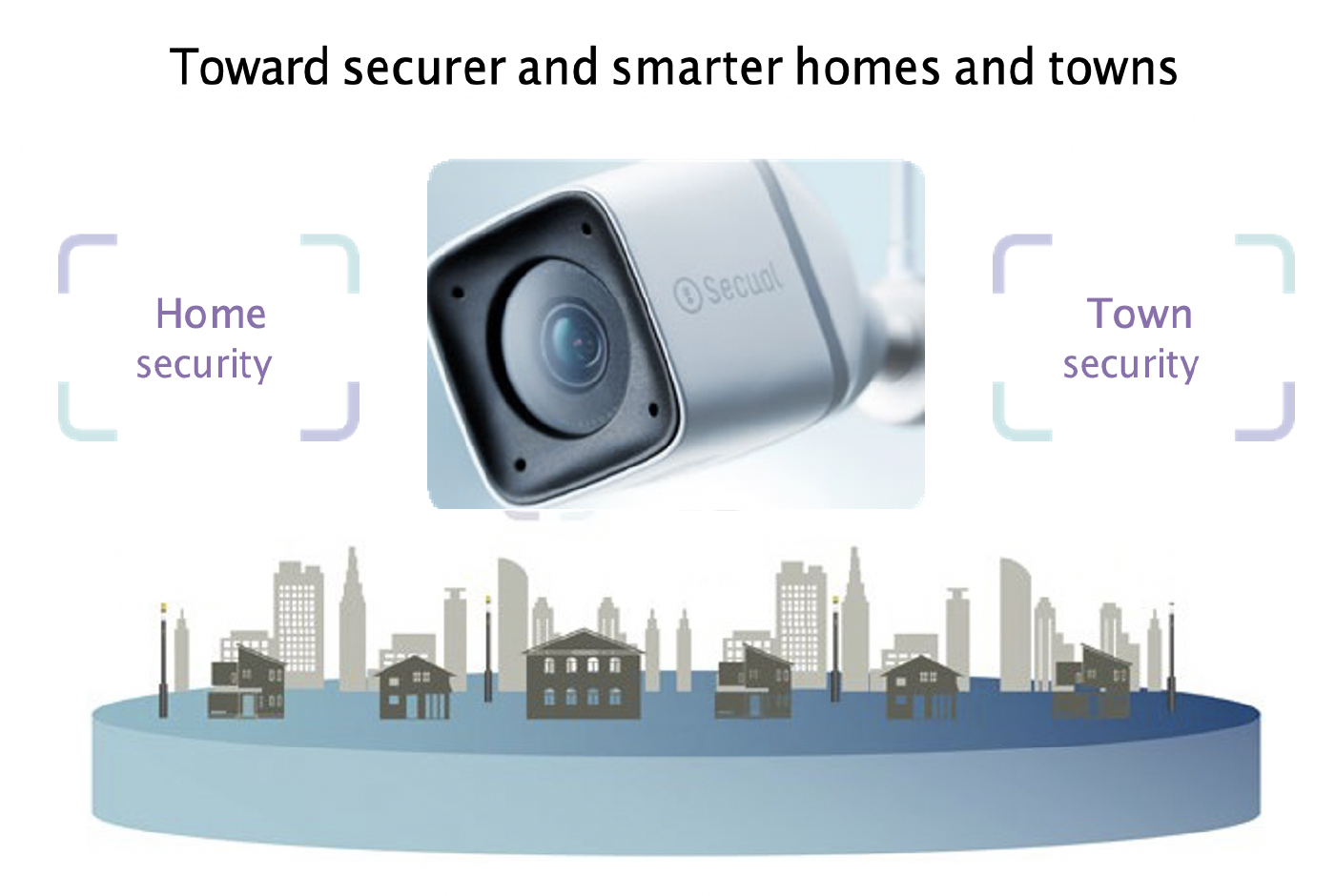
 The IoT and urban development
The IoT and urban development
 IT and education
IT and education

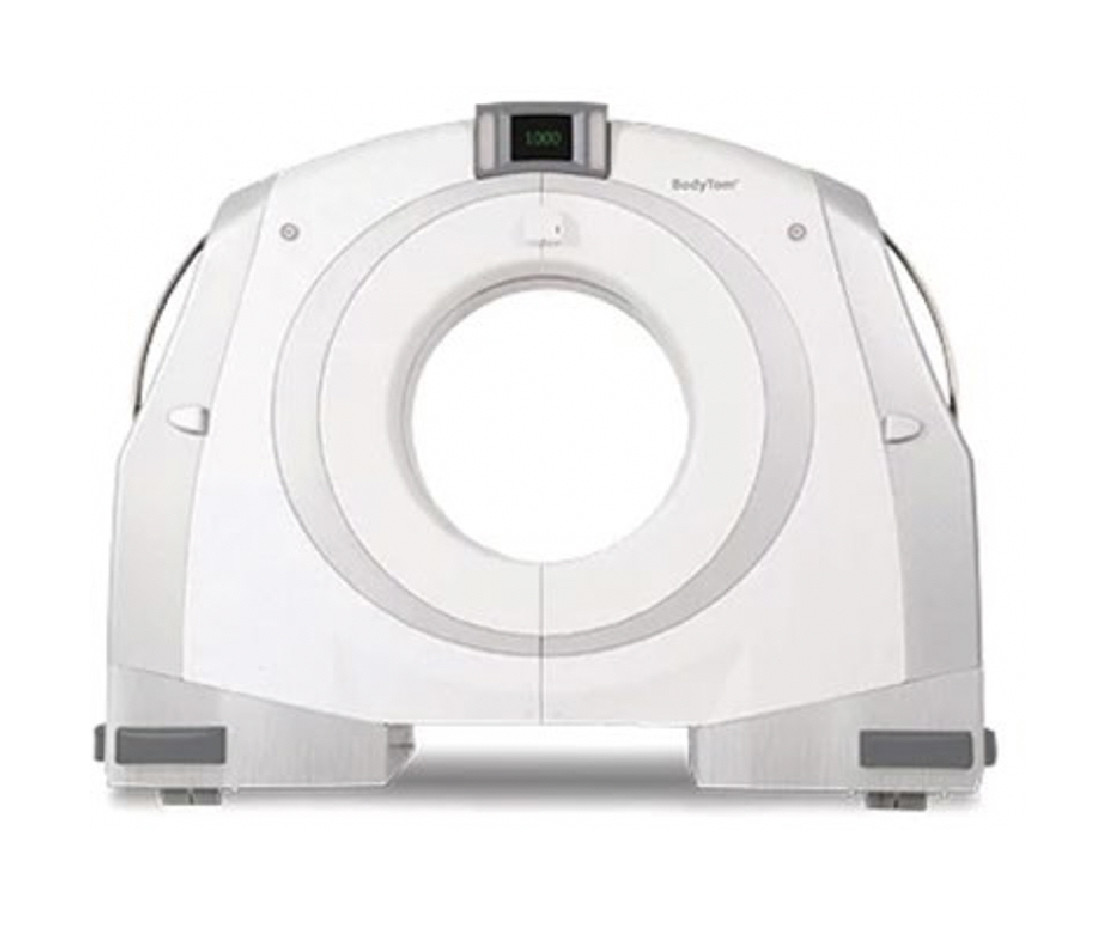
 Medical devices and QOL
Medical devices and QOL
 Effective utilization of “things”
Effective utilization of “things”
 Biomass power generation and regional contribution
Biomass power generation and regional contribution
* ORC (Organic Rankine Cycle) turbine system: Unlike conventional steam turbines that use water, this technology employs organic media such as silicon oil to generate power. As a result, steam can be generated at a lower temperature than when using water (approximately 50 degrees Celsius), making power generation more efficient.
■SDGs Contribution Award
Based on its corporate philosophy, “Everything we do is for our customers,” as outlined in its Sustainability Policy, the Kaga Electronics Group will contribute to the realization of a sustainable society by striking a balance between solving social issues and sustaining growth as a corporation through its business activities. To achieve this, this award system, established in 2022, is aimed at fostering a corporate culture in which each and every employee of the Company is encouraged and motivated to take an interest in and work toward SDGs on a daily basis. Winners are awarded shares of the Company’s stock.
In FY2024, the individual award recognized the introduction of sales of IH soldering equipment that reduces CO₂ emissions, while the group award honored various initiatives, including our sponsorship of EV buses for the Osaka-Kansai Expo and development of an app for agricultural machinery control systems to facilitate smart farming.